Deciding to undergo predictive genetic testing for an adult-onset, incurable condition such as Huntington’s disease (HD), frontotemporal dementia or familial amyotrophic lateral sclerosis (FALS) can be difficult for at-risk individuals. When an individual has a parent with one of these conditions, they have a 50% chance of having inherited the disease-causing mutation themselves. If they inherited the mutation, there is similarly a 50% chance they will pass it on to their own child. Given this risk, many at-risk individuals are interested in options that would allow them to avoid passing the condition on to a future generation. It may be surprising to hear that at-risk individuals considering a pregnancy don’t necessarily have to have genetic testing themselves in order to use fertility techniques to avoid passing the disease-causing mutation to their children. There are two different ways this can be performed: exclusion testing, which is reviewed in this article, and nondisclosure testing, which is covered in detail here.
What is exclusion testing?
Preimplantation genetic testing (PGT) for late-onset conditions like those above works the same as it does for all other single gene disorders, starting with in vitro fertilization (IVF). The typical procedure involves identifying the mutation in the at-risk parent and then directly testing for that specific mutation in the embryos. In these cases, the prospective parents learn prior to being considered a candidate for PGT whether or not they have the disease-causing mutation.
Exclusion testing, in contrast, is an indirect testing approach offered in situations where an asymptomatic person at risk for an adult-onset autosomal dominant disorder wishes to remain unaware of his/her disease status, but nonetheless does not want to pass the mutation on to a child. Exclusion testing utilizes a method known as linkage analysis. The laboratory will collect DNA samples from the parents of the at-risk individual who wishes to do PGT. Scientists can use linkage analysis to establish a unique “DNA fingerprint” for both parents – the one with the condition and the one without. Embryos from the couple desiring a pregnancy can then be tested, and the “fingerprint” each embryo inherited can be identified. Any embryos that have inherited the DNA fingerprint from the grandparent with the condition are excluded from transfer. Embryos carrying the DNA fingerprint from the unaffected grandparent are assumed to not have inherited the disease-causing mutation. In this fashion, the at-risk individual can avoid undergoing testing themselves, keeping their personal disease status unknown while ensuring their future children will not have the disorder.
For HD, one laboratory in the U.S. reports that 45% of their PGT cases utilized exclusion testing. A study conducted in Europe found that 32% of couples utilizing PGT for HD elected exclusion testing over direct mutation testing. Thus, it appears that many individuals who are at risk for HD are interested in avoiding passing the HD gene to their children, while not revealing their own HD status
Case Example
Let’s discuss a case example of how PGT with exclusion testing would work. Eliza and Eric are interested in having a child. Eliza’s mother, Leanne, has HD, but Eliza does not want to learn her disease status. Eliza has a 50% chance of inheriting the disease-causing mutation from her mother.
Humans have 46 chromosomes. There are two copies of each chromosome which are arranged into 23 pairs. We inherit one copy of each chromosome from our mother and the other copy from our father. The gene for HD is located on chromosome 4. Leanne, Eliza’s mother, has one copy of the mutation that causes HD and one normal copy. Eliza’s father presumably has two normal copies since he has no personal or family history of HD. Because Eliza has a 50% chance of inheriting the HD mutation from her mother, she decides to undergo IVF/PGT with exclusion testing to reduce the risk of passing on the disease-gene to her child.
In order to develop a test, the genetics lab will need DNA samples from Eric, Eliza, Leanne and Larry. Eliza and Eric will undergo in vitro fertilization to create embryos, which will be biopsied and sent to the lab for exclusion testing. The laboratory will create a DNA fingerprint to distinguish chromosomes that come from Leanne versus chromosomes that comes from Larry.
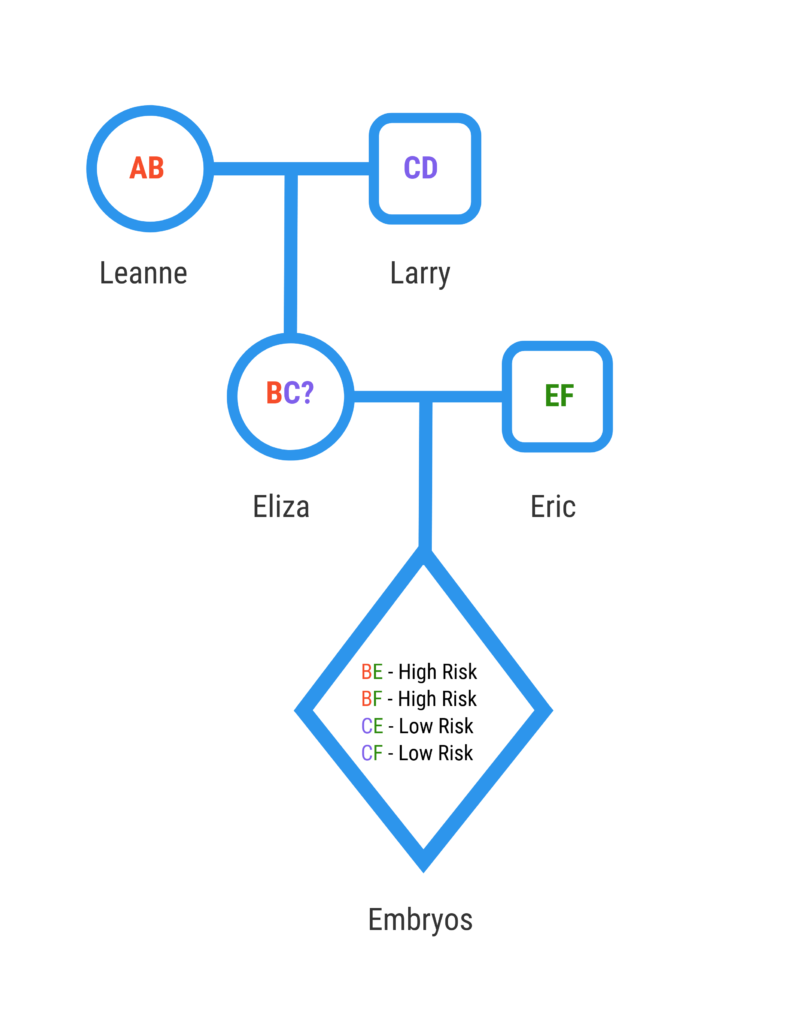
We can assume that each embryo will have a normal chromosome from Eric. Eliza has inherited one chromosome from Larry and the other chromosome from Leanne. We do not know if she inherited the chromosome from Leanne that has the HD mutation since we did not do direct mutation testing. Eliza has a 50% chance of passing on the chromosome she inherited from Larry and a 50% chance of passing on the chromosome she inherited from Leanne. The lab will apply DNA fingerprinting to see which chromosomes the embryos inherited. Embryos that inherited one of Leanne’s copies are classified as “at risk”, since there is a 50% chance that they have the chromosome with the HD mutation. These embryos are excluded from being transferred.
Eliza can hold on to the higher risk embryos in case she decides to get direct mutation testing for HD in the future. If she finds that she inherited Leanne’s chromosome without the mutation, she can safely transfer the embryos with Leanne’s DNA fingerprint.
What should I consider before undergoing exclusion testing?
There are several difficult questions that potential parents should consider before undergoing exclusion testing. Rachael Cabey, lead laboratory genetic counselor at Cooper Genomics, always asks patients considering exclusion testing if the results of their disease status would affect their decision to have children. It is worth considering the logistics of parenting if the at-risk person were to begin developing symptoms during their child’s early life.
Cabey also notes exclusion testing reduces the number of transferable embryos, which can reduce the chance of pregnancy. Roughly half of the embryos will be classified as higher risk. If the at-risk parent does have an underlying mutation, all of the embryos labelled higher risk will have inherited the mutation. But, if the at risk parent does not have the underlying mutation, none of the embryos will have an increased risk for the disease. Thus, potentially healthy embryos are being excluded from transfer.
Additionally, exclusion testing can generally only be performed if at least one parent of the at-risk individual can provide a DNA sample. Therefore, if both parents are deceased or unavailable, then exclusion testing likely cannot be performed. In this case, individuals who want to prevent the potential for passing on the disease mutation to their child can choose to have direct testing. If a mutation is identified, they can undergo IVF with PGT, but will be aware of their own disease status.
Individuals that are making decisions about their own genetic testing and their reproductive options for late-onset conditions may wish to consider speaking with a genetic counselor with experience in reproductive genetics to help navigate these difficult questions.

Nisha is employed by ORM Fertility as a Genetic Services Coordinator where she works with clinical and administrative teams and patients to seamlessly integrate genetic services into the ORM patient experience throughout the treatment process. She has a background in genetics research and advocacy, and brings her passion for learning to the work she does. Nisha obtained her Bachelor’s degree in Biology (BA) from the University of Portland. During her undergraduate studies, she worked with adolescents with disabilities along with conducting research at a cytogenetics laboratory. Nisha will be attending graduate school for Genetic Counseling in the Fall of 2019.
